SMATCH
Statistical and AI based Methods for Advanced Clinical Trials CHallenges in Digital Health
SMATCH is a consortium (2023-2029) from the PEPR Santé Numérique (project n°22-PESN-0003) headed by INRIA-INSERM, part of the France 2030 initiative. It gathers researchers from different institutions on the development of Statistical and AI based Methods for Advanced Clinical Trials CHallenges in Digital Health.
General Presentation
Health-related interventions and their evaluation have been revolutionized by advances in biotechnology and digitization and expectations are high. To support the acceleration of drug development and other medical interventions through the use of artificial intelligence (AI) and data from a variety of sources, there is a need for a clear understanding of the appropriateness of this use and for robust trial evaluation methods. The need to adapt the methods used and to propose robust innovative approaches to clinical trials is increasingly critical, and for example, the proliferation of new digital medical devices using AI alone requires specific methods to evaluate their ultimate impact on health.
The objective of the SMATCH project is therefore to develop and apply statistical and AI-based methods with the ultimate goal of accelerating the development of medical interventions (drugs and digital medical devices) during their evaluation in clinical trials based on the following assumptions:
- The use of information generated in preclinical studies (animal studies, organoids, in silico studies) combined with adaptive designs should help the early phases of development;
- The integration of multi-source data including real-world and in silico data should help to complete trials;
- Specific adaptive designs should be defined for the evaluation of digital medical devices based on learning algorithms.
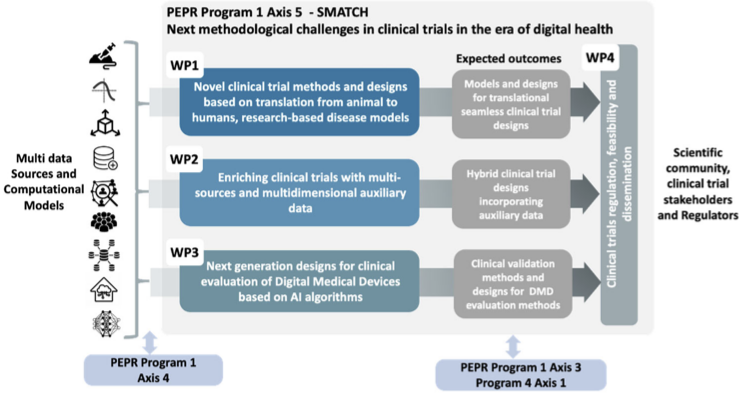
To this end, SMATCH is structuring its research around four operational workpackages:
- Research of new clinical trial methods and designs based on the translation of research-based disease models from animals to humans;
- Development of new approaches for enriching clinical trials with multi-source and multi-dimensional ancillary data;
- Development of next generation designs for clinical evaluation of digital medical devices based on artificial intelligence algorithms;
- Evaluation with regulatory authorities and end-users of the regulatory impact and feasibility of innovative methods for clinical trials proposed for widespread use.
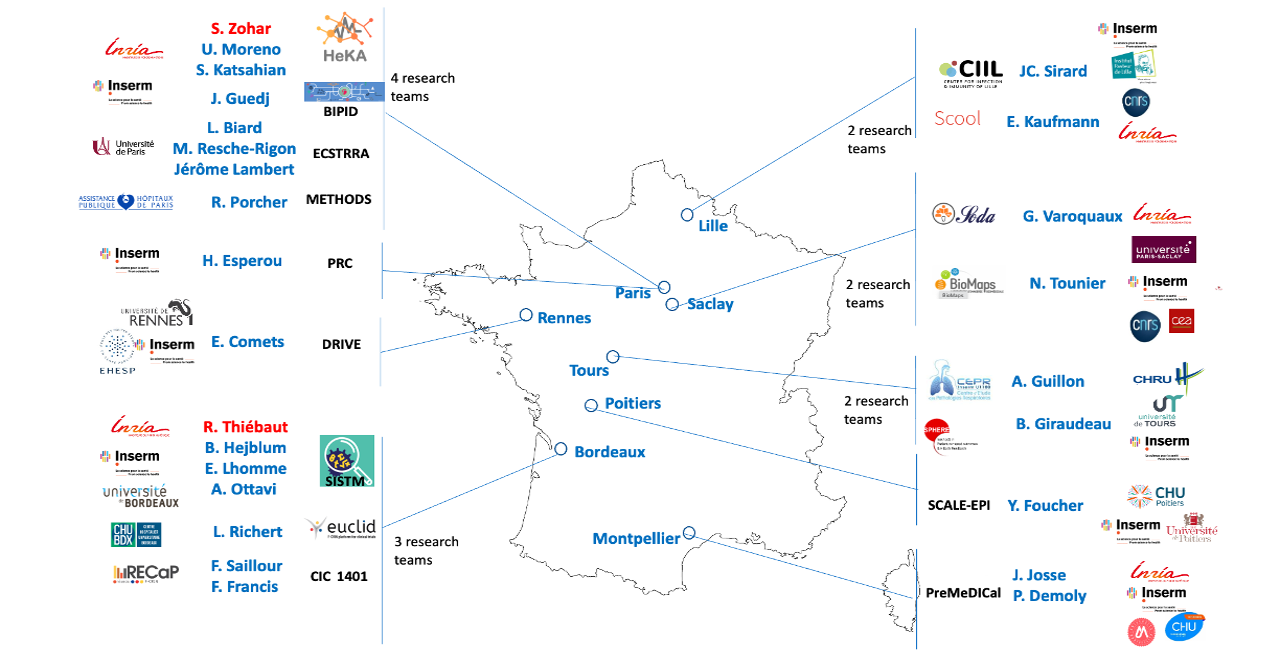
The consortium is made up of 16 teams, mainly from Inria and Inserm research centers recognized in this field, bringing a unique and complementary expertise in data sciences and AI applied to health problems and specifically to clinical trials. In addition, links with the regulatory bodies involved are already established within the consortium (e.g. HAS) and outside (e.g. EMA). Moreover, all methodological projects are applied to ongoing health studies in various fields. Finally, many connections exist with the other axes of the PEPR Digital Health and more generally with the projects carried out within the framework of the digital health acceleration strategy. Thus, by providing innovative and adapted methodological tools that will have already been applied in a real context, we hope to participate in the acceleration of clinical research leading to major societal and economic impacts.
WP1: Novel clinical trial methods and designs based on translation from animal to humans, research-based disease models

The global objective is to propose a methodological framework for accelerating drug development through mechanistic translational and bridging approaches, in order to refine and optimize animal experiments, gain information on PK/PD, and enable dose-finding and early selection for the Phase I/II clinical trials.
-
Build physiologically-based PK (PBPK) and statistical methods that provide results that are robust to model uncertainties to (1) predict clinical efficacy of antivirals and vaccines using the large amounts of preclinical data that have already been generated in COVID19 (2) develop a first-in- man exploratory clinical trial design using microdosing PET data
-
Develop early-phase clinical trial designs based on Bayesian adaptive approaches for exploration and down-selection among multiple potential regimens based on multivariate outcomes applied to vaccine development and drug resistant infections
WP2: Enriching clinical trials with multi-sources and multidimensional auxiliary data

Enriching clinical trials with multi-sources and multidimensional auxiliary data: to develop the next generation of methods to combine high-throughput, high-dimensional individual and ancillary data in clinical trials and to evaluate their benefit.
Objectives:
- Integrate internal data, i.e., supplementing the clinical trial data with additional patient information by integrating high-dimensional omics data
- Integrate external multi-sources information (i.e. observational data), by
- selecting the relevant data to be used,
- estimating heterogeneity and generalizing the treatment effects in other population, and
- coping with information loss of interrupted clinical trials and avoiding research waste.
- Integrate auxiliary data (synthetic, observational, etc) to complete a partial
clinical trial, i.e. emulating a trial by
- generation of in-silico or synthetic patients for clinical trials, and
- from single arms studies toward generalization of finding.
WP3: Next generation designs for clinical evaluation of Digital Medical Devices based on AI algorithms

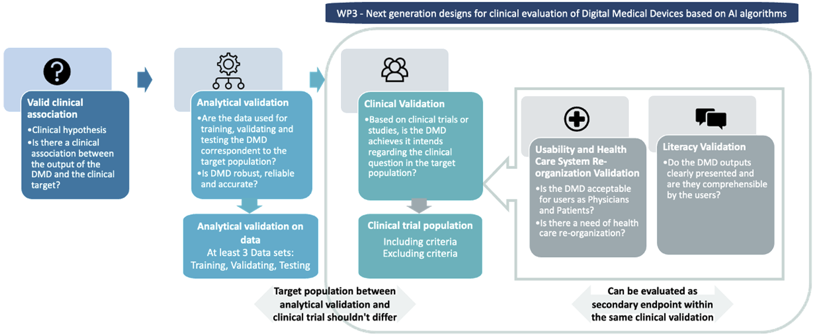
WP3 will focus on design aspects needed for the clinical validation step. Indeed, our aim is not to focus on the analytical validation — for which specific methods will be proposed in the AI PEPR regarding robustness and accuracy of AI based algorithms — but rather on proposing clinical trial or RWD (real world data) study designs, in which the patient’s outcomes are always primary that could be associated with secondary endpoints related to acceptability, literacy and Care System Management re-organization. We will also, work on post-CE marking as it requires specific considerations regarding the physical (if the DMD is associated with a hardware), an analytical validation as well as benefit-risk evaluation.
- Propose novel endpoints associated with health care reorganization and digital biomarkers (i.e., quantifiable physiological and behavioural data that are collected and measured by means of digital devices) for the evaluation of disease DMDs
- Develop and propose randomized adaptive clinical study designs for DMDs allowing maximizing response and minimizing risks for software or software associated with hardware used by patients.
- Develop approaches to evaluate the effectiveness of DMDs for prevention and disease monitoring using real world data
- Propose new methodology for assessing modifications or corrections of devices (algorithms, software…) that lowers the costs of evaluation while maintaining validity and control for small modification.
- Assess and provide methods for regulatory needs in terms of evaluation of DMDs
WP4: Clinical trials’ regulation, feasibility and dissemination

The objective of WP4 is to present and discuss the results and deliverables provided by every workpackage of the SMATCH project to the stakeholders (ANSM as regulatory authority, patient associations, experts in ethics, legal sponsors and clinical trials units) to assess acceptability and feasibility from the regulatory, ethical and operational perspectives. This will be done throughout the organization of 4 workshops with the stakeholders and extensive publications of our results.
- Critically assess the specific regulatory modalities of the novel designs and methods proposed by WP1-WP3 along the regulatory process including the reporting
- Critically assess the operational feasibility of the novel designs and methods proposed by WP1- WP3
- Provide an ethical perspective on the novel designs and methods proposed by WP1-WP3
- Disseminate the results of this PEPR axis
